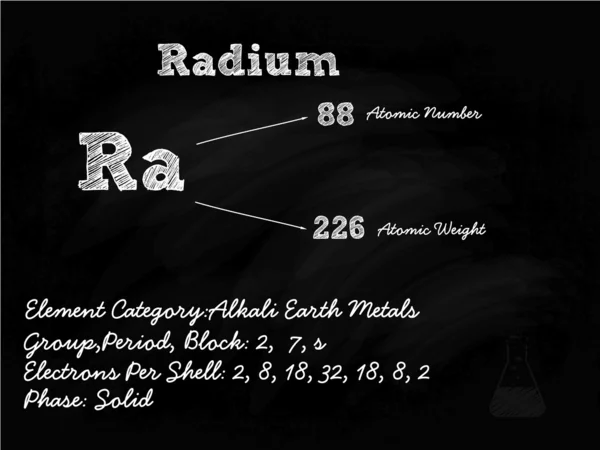
Tablicu Mendeleeva V Vektore
S1 boot fastboot driver win8 windows 7. Dmitri Mendeleev Between 1859 and 1861, he worked on the and the workings of the in. Later in 1861, he published a textbook named Organic Chemistry. This won him the Demidov Prize of the Petersburg Academy of Sciences. On 4 April 1862 he became engaged to Feozva Nikitichna Leshcheva, and they married on 27 April 1862 at 's church in Saint Petersburg (where he taught).
Aug 21, 2016 - http://studentdelo.ru/tablica-mendeleeva/. -prazdnovat-gol-ne-v-tot-sektor.html 2014-04-24T08:42:16+04:00 weekly 0.9.
Mendeleev became a professor at the and in 1864, and 1865, respectively. In 1865 he became Doctor of Science for his dissertation 'On the Combinations of Water with Alcohol'. He achieved in 1867 at St. Petersburg University and started to teach inorganic chemistry, while succeeding Voskresenskii to this post. And by 1871 he had transformed Saint Petersburg into an internationally recognized center for chemistry research. In 1876, he became obsessed with Anna Ivanova Popova and began courting her; in 1881 he proposed to her and threatened suicide if she refused. His divorce from Leshcheva was finalized one month after he had married Popova (on 2 April ) in early 1882.
Even after the divorce, Mendeleev was technically a; the required at least seven years before lawful remarriage. His divorce and the surrounding controversy contributed to his failure to be admitted to the Russian Academy of Sciences (despite his international fame by that time). His daughter from his second marriage, Lyubov, became the wife of the famous Russian poet.
His other children were son Vladimir (a sailor, he took part in the notable ) and daughter Olga, from his first marriage to Feozva, and son Ivan and twins from Anna. Though Mendeleev was widely honored by scientific organizations all over Europe, including (in 1882) the from the of London (which later also awarded him the in 1905), he resigned from Saint Petersburg University on 17 August 1890. He was elected a, and in 1893 he was appointed director of the Bureau of Weights and Measures, a post which he occupied until his death. Mendeleev also investigated the composition of petroleum, and helped to found the first in Russia. He recognized the importance of petroleum as a feedstock for.
He is credited with a remark that burning petroleum as a fuel 'would be akin to firing up a kitchen stove with bank notes'. In 1905, Mendeleev was elected a member of the. The following year the recommended to the Swedish Academy to award the for 1906 to Mendeleev for his discovery of the periodic system. The Chemistry Section of the Swedish Academy supported this recommendation.
Like any skill, you really learn BPMN only by doing it, working through the creation of diagrams that clearly express your intended meaning. Bruce silver bpmn method and style pdf download.
The Academy was then supposed to approve the Committee's choice, as it has done in almost every case. Unexpectedly, at the full meeting of the Academy, a dissenting member of the Nobel Committee,, proposed the candidacy of whom he favored., although not a member of the Nobel Committee for Chemistry, had a great deal of influence in the Academy and also pressed for the rejection of Mendeleev, arguing that the periodic system was too old to acknowledge its discovery in 1906. According to the contemporaries, Arrhenius was motivated by the grudge he held against Mendeleev for his critique of Arrhenius's. After heated arguments, the majority of the Academy chose Moissan by a margin of one vote.
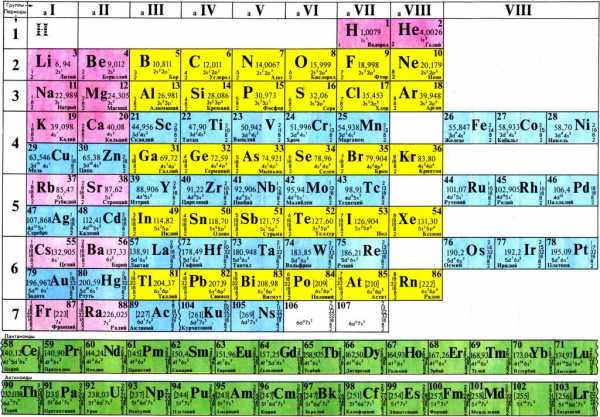
The attempts to nominate Mendeleev in 1907 were again frustrated by the absolute opposition of Arrhenius. In 1907, Mendeleev died at the age of 72 in from. His last words were to his physcian: 'Doctor, you have science, I have faith,' which is possibly a quote. The crater on the, as well as element number 101, the radioactive, are named after him. Periodic table. Sculpture in honor of Mendeleev and the periodic table, located in, Slovakia In 1863 there were 56 known with a new element being discovered at a rate of approximately one per year. Other scientists had previously identified periodicity of elements.